38 phase diagram with labels
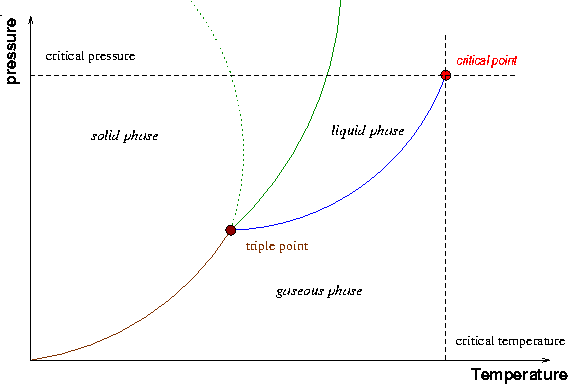
SOLVED:Draw a generic phase diagram and label its important ... VIDEO ANSWER: Okay, so Chapter eight, no Chapter eleven, Section thirty four SS to draw generic phase diagram and label. It's important features. phase diagram | physics | Britannica phase diagram, graph showing the limiting conditions for solid, liquid, and gaseous phases of a single substance or of a mixture of substances while undergoing changes in pressure and temperature or in some other combination of variables, such as solubility and temperature. The Figure shows a typical phase diagram for a one-component system (i.e., one consisting of a single pure substance ...
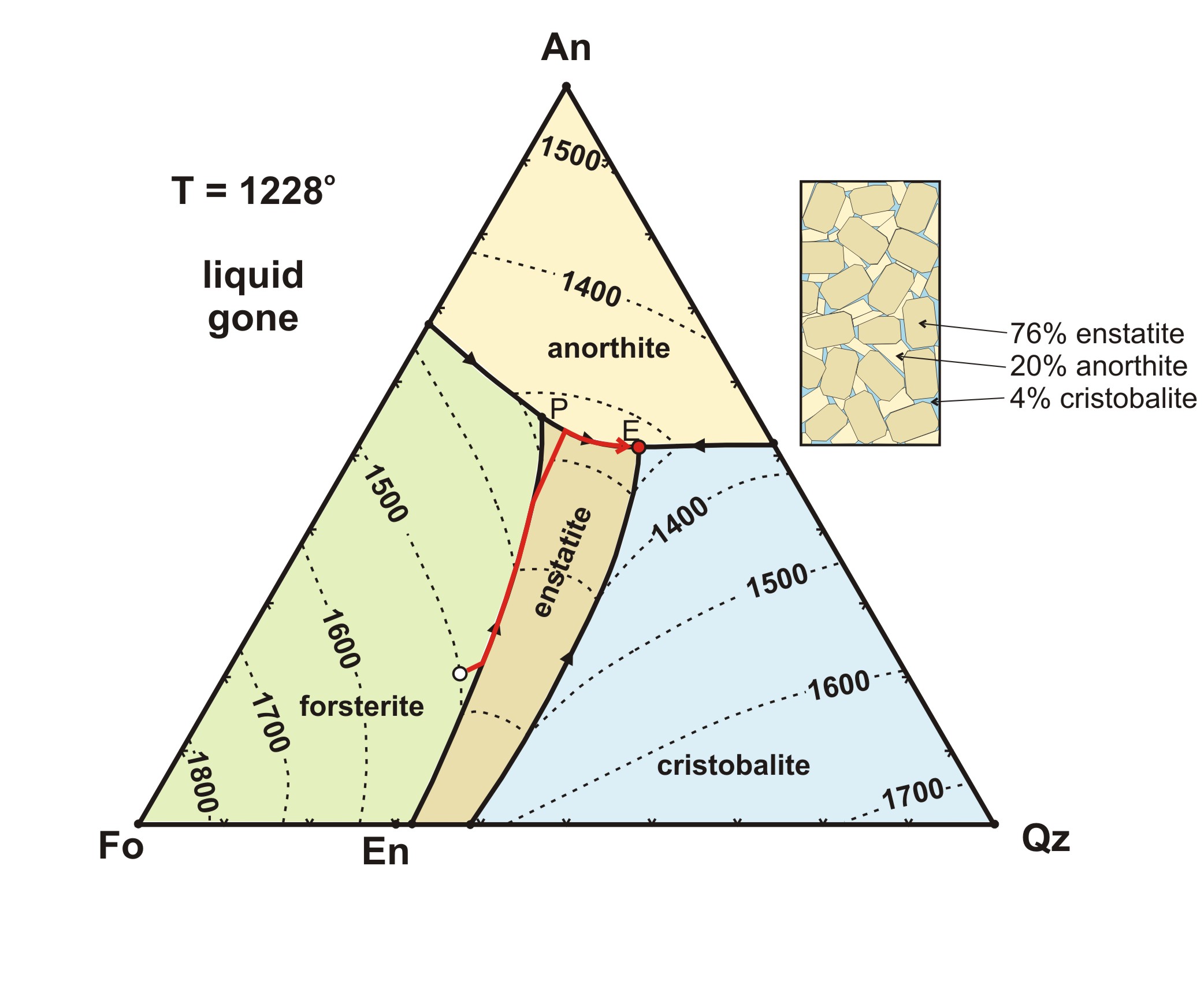
Phase Diagram: Meaning and Types | Material Engineering A phase diagram is also called an equilibrium or constitutional diagram. It shows the relationship between temperature, the compositions and the quantities of phases present in an alloy system under equilibrium conditions. When temperature is altered many microstructure develop due to phase transformation.
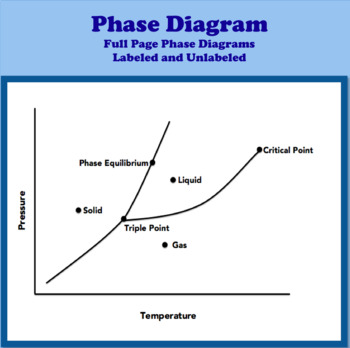
Phase diagram with labels
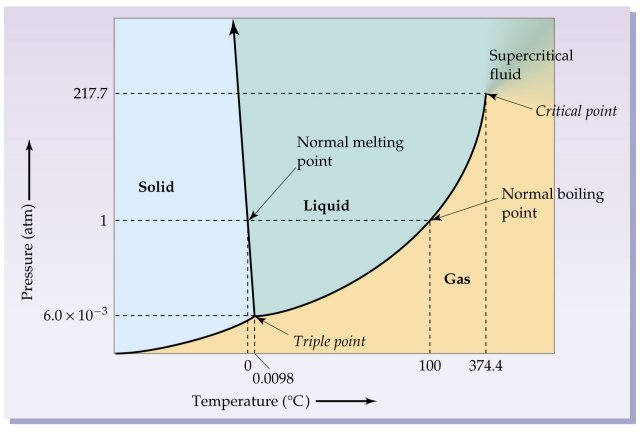
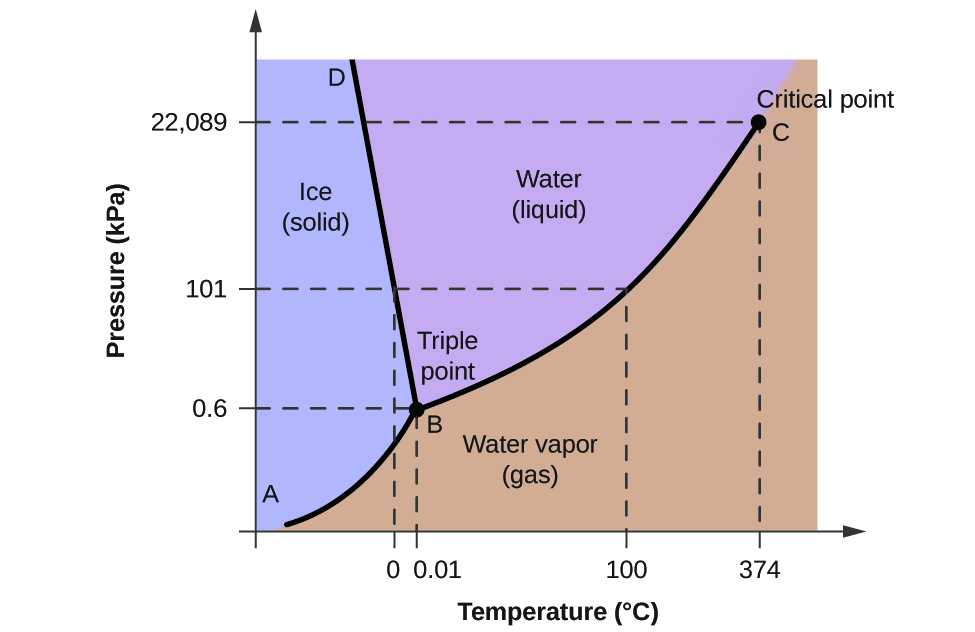
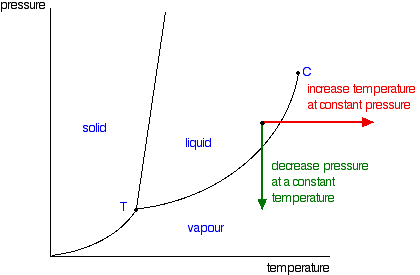
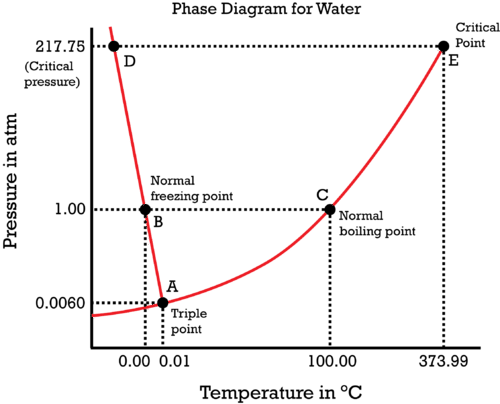
Phase Diagrams - Purdue University You can therefore test whether you have correctly labeled a phase diagram by drawing a line from left to right across the top of the diagram, which corresponds to an increase in the temperature of the system at constant pressure. When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas. Phase Diagram for Water | Chemistry for Non-Majors - Course Hero Notice one key difference between the general phase diagram and the phase diagram for water. In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. ... Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the gas phase are moving very ... Phase Diagrams · Chemistry (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram.
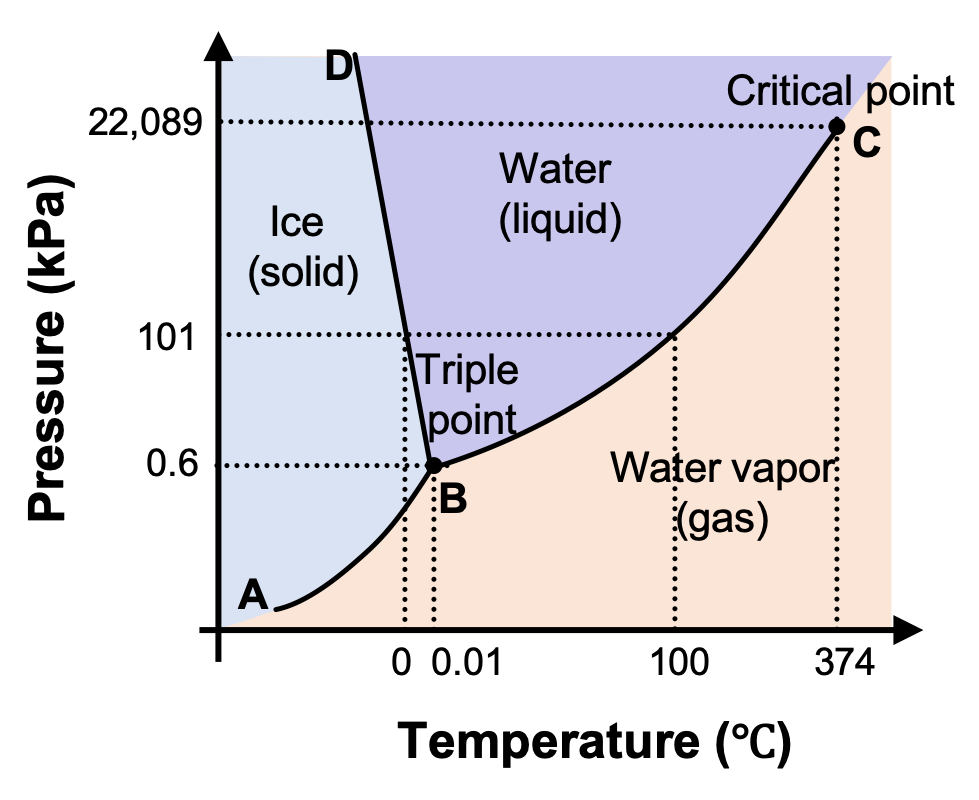
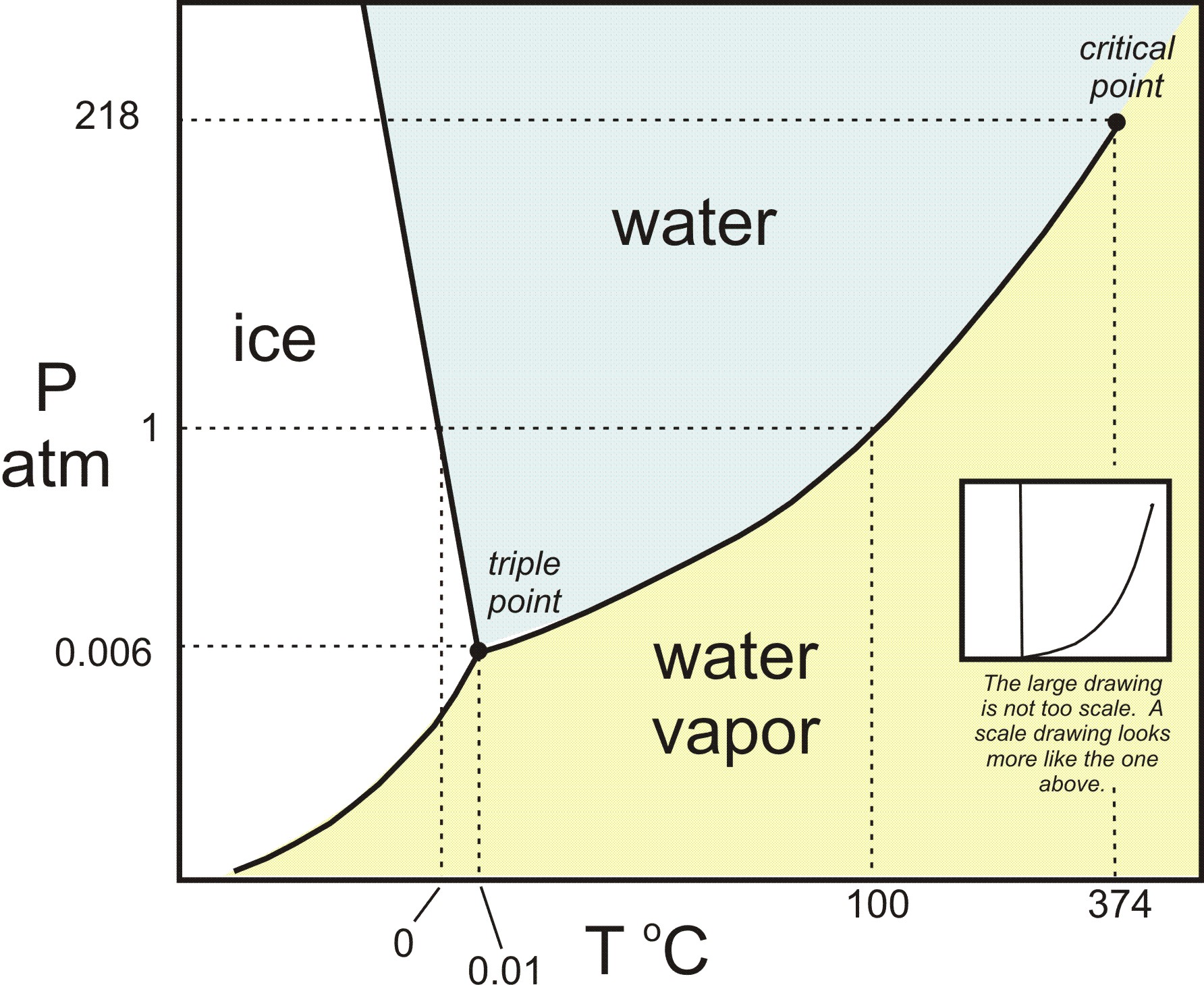
Phase diagram with labels. Phase Diagrams | Chemistry: Atoms First - Lumen Learning A phase diagram combines plots of pressure versus temperature for the ... The solid-liquid curve labeled BD shows the temperatures and pressures at which ... 10.4 Phase Diagrams - General Chemistry 1 & 2 (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. Iron-Carbon Phase Diagram Explained [with Graphs] - Fractory This iron carbon phase diagram is plotted with the carbon concentrations by weight on the X-axis and the temperature scale on the Y-axis. Iron crystal structures explained The carbon in iron is an interstitial impurity. The alloy may form a face centred cubic (FCC) lattice or a body centred cubic (BCC) lattice. Phase Diagrams - Chemistry - University of Hawaiʻi We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
1. Draw the PV phase diagram and label phases and | Chegg.com Draw the PV phase diagram and label phases and critical point on the phase diagram; Question: 1. Draw the PV phase diagram and label phases and critical point on the phase diagram. This question hasn't been solved yet Ask an expert Ask an expert Ask an expert done loading. Show transcribed image text Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams Phase diagrams (video) | States of matter | Khan Academy And there are many forms of phase diagrams. This is the most common form that you might see in your chemistry class or on some standardized test, but what it captures is the different states of matter and when they transition according to temperature and pressure. This is the phase diagram for water. So just to understand what's going on here ... Phase Diagram Manipulate and Refresh Window - FactSage.com phases and labels; iso-activity lines; tie lines - isothermal diagram; polythermal projection; aqueous diagram. The screenshot shows all 5 categories ...
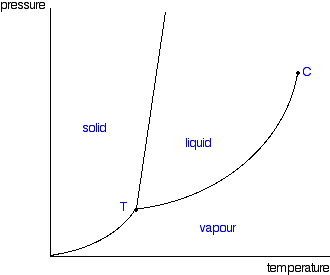
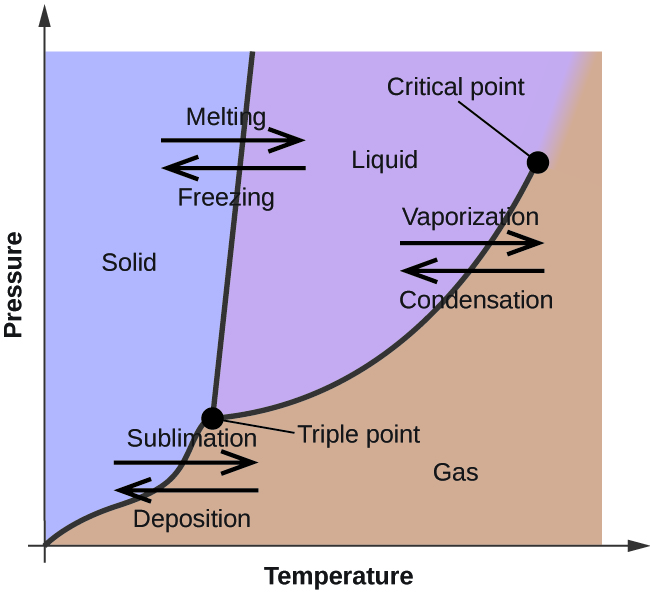
phase diagrams of pure substances - chemguide A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. Phase Diagrams | Boundless Chemistry | | Course Hero A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely. 10.4: Phase Diagrams - Chemistry LibreTexts Sep 22, 2022 ... Phase diagrams are combined plots of three pressure-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas. These curves ... Phase Diagram | Explanation, Definition, Summary & Facts A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any point at the curve lines shows the equilibrium between two phases. Phase diagram explanation
Phase Diagrams - Chemistry LibreTexts Sep 10, 2022 ... Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A ...
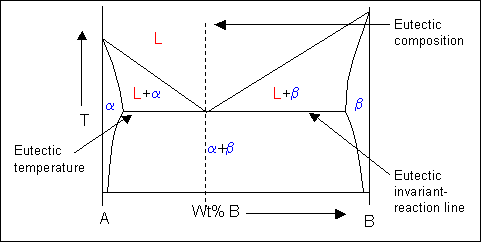
How to label a blank phase diagram - YouTube Worked example problem solution of how to label single and two phase regions on an unlabeled phase diagram. Also, how to recognize key reactions such as eute...
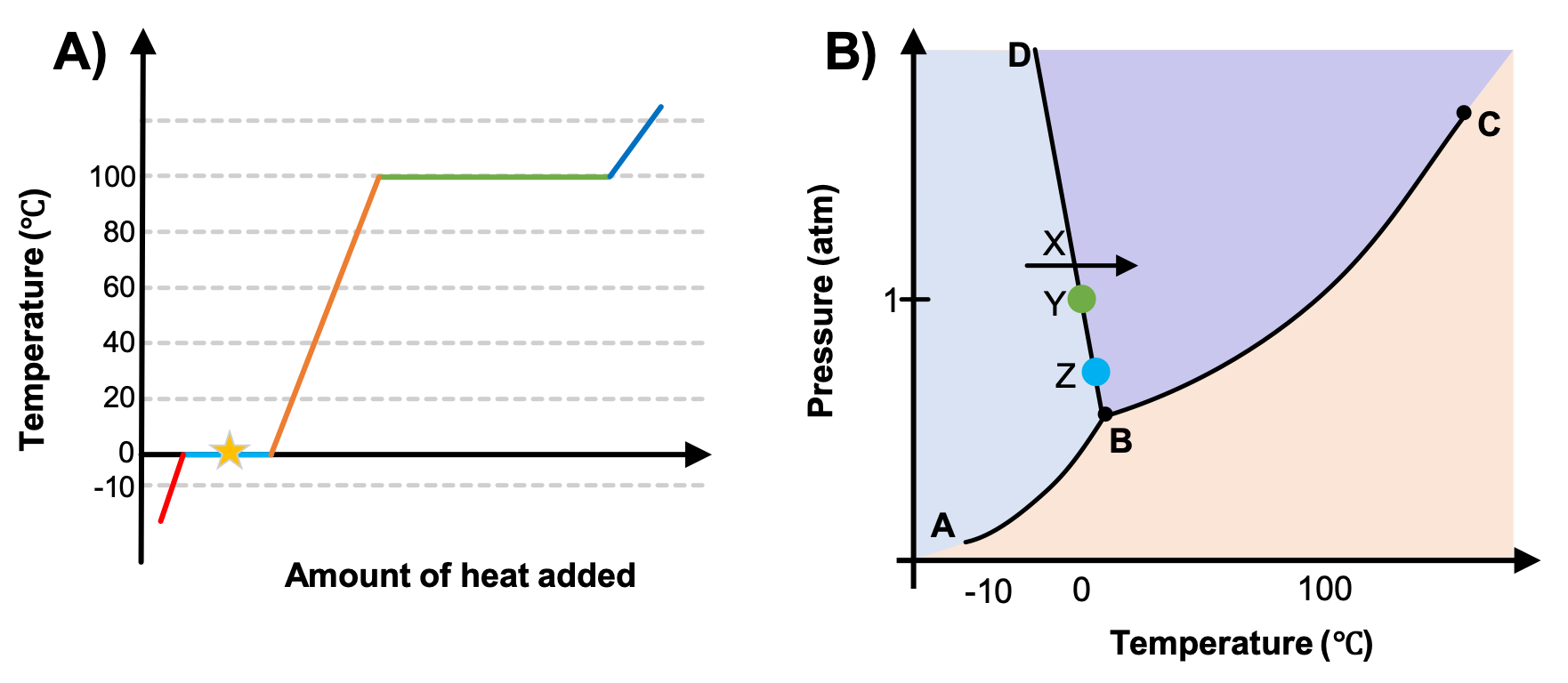
Labeling Phase Change Diagrams | Chemistry | Study.com Steps for Labeling Phase Change Diagrams. Step 1: Locate the triple point on the pressure vs. temperature phase diagram. This should look like the intersection of the letter Y . Step 2: Follow the ...
What's New in Phase Diagram - FactSage The resulting calculated diagram is a true CaCl 2 - (NaF)2-CaF 2 - (NaCl)2 reciprocal system with a 'square frame' and the corners labelled accordingly. Reciprocal diagrams are not limited to molten salts Here is the (CaO)3-Al 2 O 3 -Ca 3 N 2 - (AlN)2 reciprocal diagram with data taken from the FTOxCN database.
Phase Diagrams - Phases of Matter and Phase Transitions - ThoughtCo A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries.
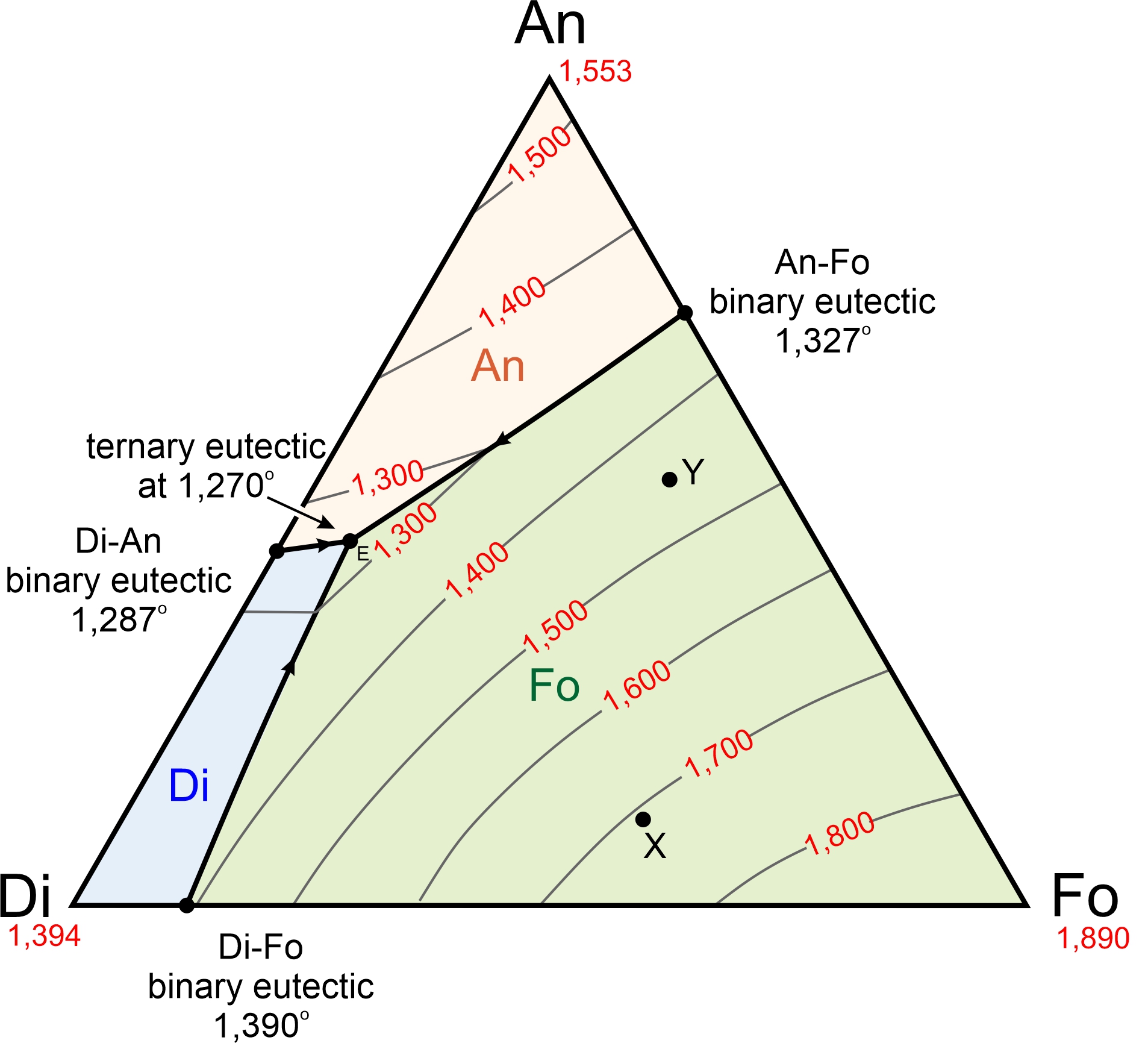
Prediction of Phase Diagrams and Associated Phase Structural Properties ... The output features are derived from the 14428 parsed phase diagram labels from the collection of phase diagrams including binary and ternary diagrams. Each region of the phase diagram can be labeled or unlabeled. When labels are detected, we distinguish between the presence or absence of amorphous, gas, liquid, and solid phases. ...
Phase Diagrams - University of Washington Phase Diagrams (Reference: Chapter 9, Callister) The average (2.8) student will be able to: ... (100% solid solution) phase diagram and label the regions of the diagram. for an isomorphous system describe the nature of the solid phase or phases and the liquid phase for a given overall composition.
Anaphase: Definition, Checkpoints, Diagram, and Examples The chromosomes are aligned in the equatorial plate. Anaphase: Chromosomal split forms daughter chromatids; travels to the opposite poles. The chromosomes are V - Shaped as they are dragged to the opposite sites. Telophase: Microtubules disappear and chromosomes decondense to chromatin mass. Nuclear envelope starts to form.
Label The Phase Changes Shown In The Diagram Below 27+ Pages ... Label the following diagram with the appropriate sequence of events to assess your knowledge of the major controls of the secretion of prolactin and. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. 564 9th and earlier. Assign the appropriate labels to the phase diagram shown below.
How to Label a Phase Diagram | Chemistry | Study.com Vocabulary for Labeling a Phase Diagram A solid is a phase of matter characterized by having a fixed and uniform arrangement of its particles and a very low energy system. A liquid is also a phase...
15 3c Labeling a typical simple phase diagram - YouTube Feb 5, 2018 ... ALEKS 15.3 Gases, Liquids, and Solids - Phase Change. 15 3c Labeling a typical simple phase diagram. 4,899 views4.8K views. Feb 5, 2018.
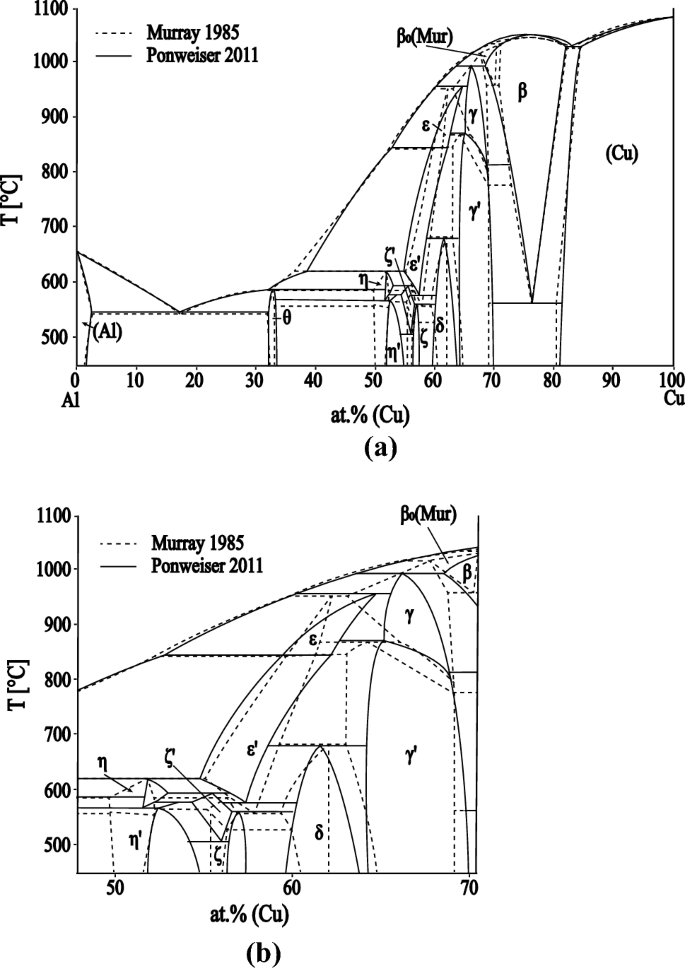
PDF Chapter 9: Phase Diagrams - Florida International University Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
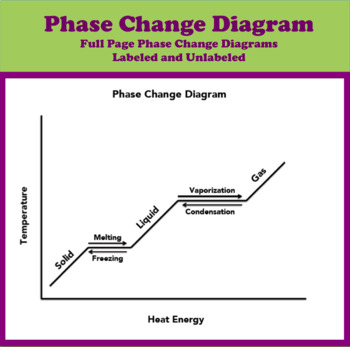
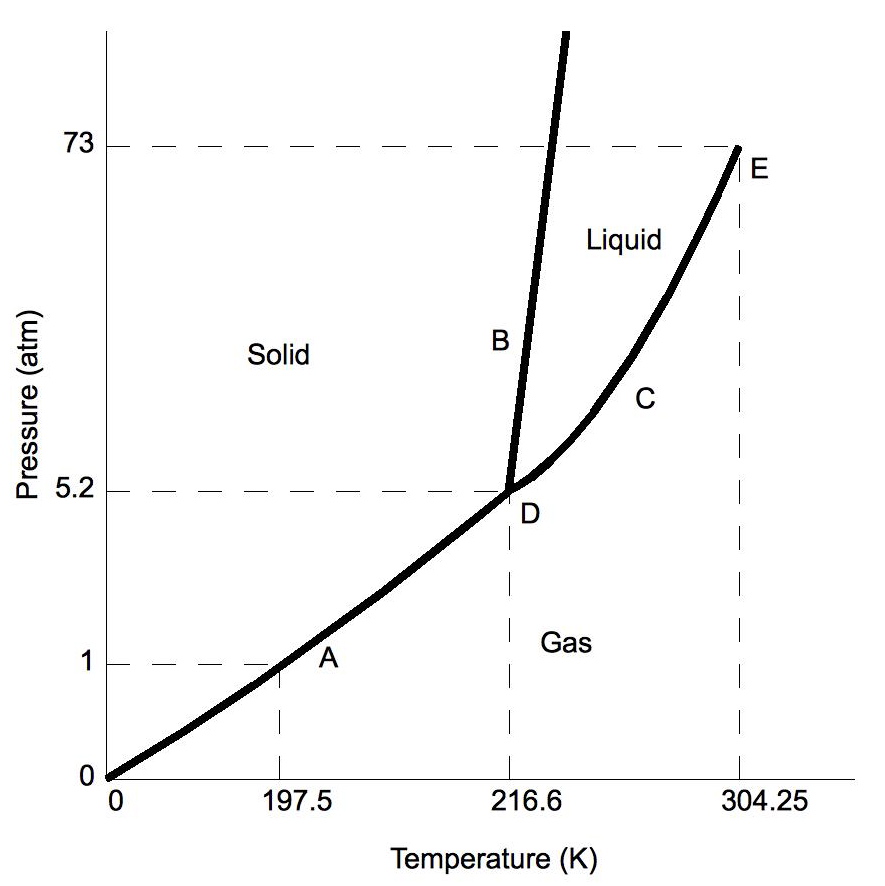
PDF Phase Diagrams States of Matter and Phase Changes Terminology of Phase Diagrams Triple Point The triple point is the location on a phase diagram at which all three lines which divide the three states of matter meet. At this point, all three states of matter may exist at the same time. What is the pressure and temperature for the
How to label a blank phase diagram - YouTube Phase diagrams are a super helpful resource for materials scientists. Labeling them can be challenging, but, fortunately, there are some simple rules to follow. The top portion will be liquid, the...
What Is a Phase Diagram? - ThoughtCo A phase diagram is a chart showing the thermodynamic conditions of a substance at different pressures and temperatures. The regions around the lines show the phase of the substance and the lines show where the phases are in equilibrium. Parts of a Phase Diagram Typically, a phase diagram includes lines of equilibrium or phase boundaries.
Phase Diagrams - Highland The triple point of naphthalene is 80°C at 1000 Pa. Use these data to construct a phase diagram for naphthalene and label all the regions of your diagram. Argon is an inert gas used in welding. It has normal boiling and freezing points of 87.3 K and 83.8 K, respectively. The triple point of argon is 83.8 K at 0.68 atm. Use these data to ...
Phase Diagram of Water - Explanation and Diagrammatic ... - BYJUS A phase diagram is a graphical representation of the various phases of a substance or mixture of substances that coexist in thermodynamic equilibrium, and undergo phase changes under different working conditions, such as temperature, pressure, or volume. The water system is divided into three phases: ICE (S), WATER (L), and WATER VAPOUR (G)
Phase Diagrams · Chemistry (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram.
Phase Diagram for Water | Chemistry for Non-Majors - Course Hero Notice one key difference between the general phase diagram and the phase diagram for water. In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. ... Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the gas phase are moving very ...
Phase Diagrams - Purdue University You can therefore test whether you have correctly labeled a phase diagram by drawing a line from left to right across the top of the diagram, which corresponds to an increase in the temperature of the system at constant pressure. When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas.
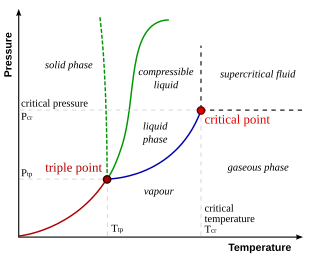




:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)














Post a Comment for "38 phase diagram with labels"